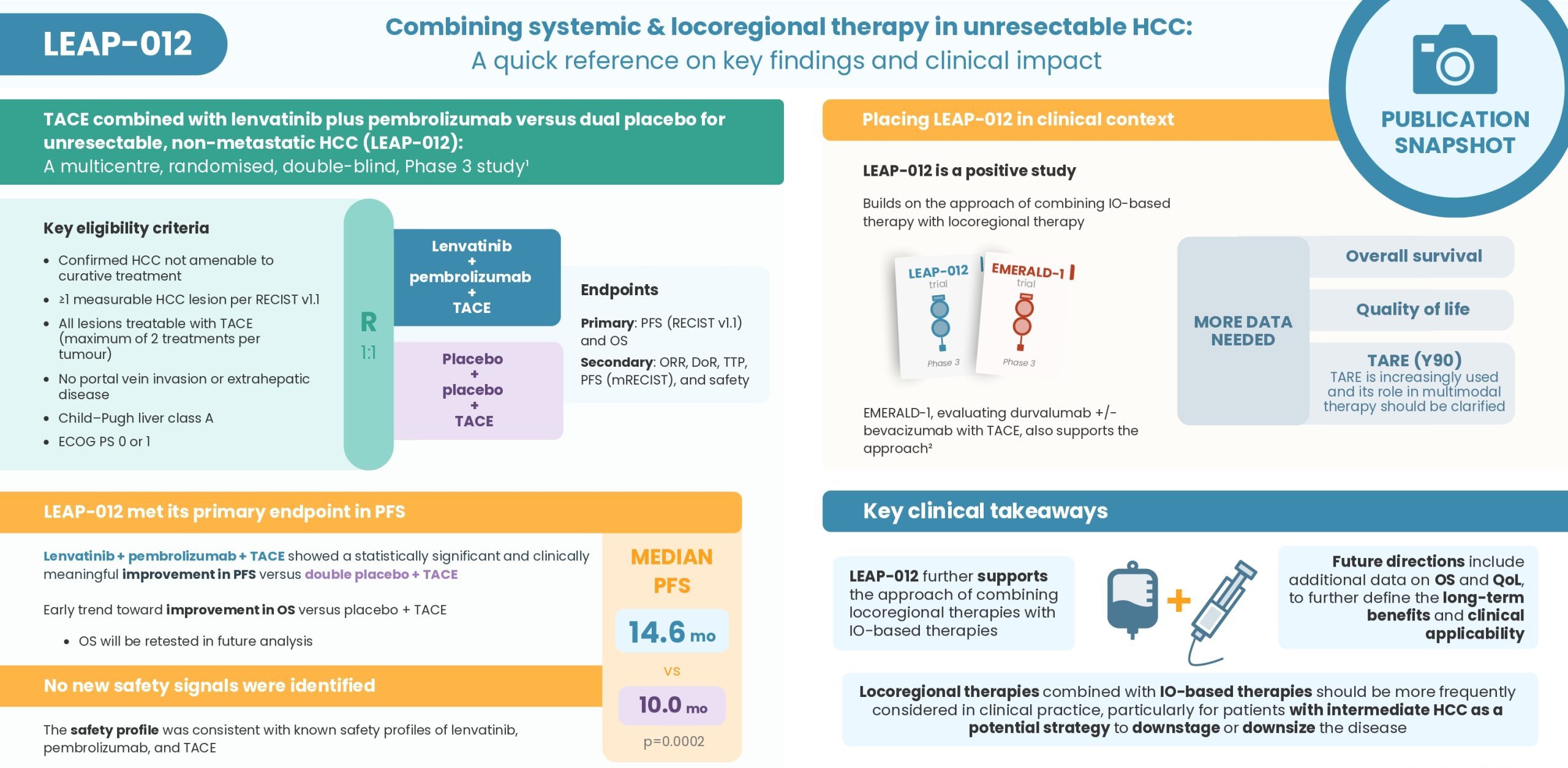
Discover insights from the Phase 3 LEAP-012 study, evaluating lenvatinib + pembrolizumab + TACE versus TACE alone in intermediate-stage HCC. In this short expert video, Prof. Arndt Vogel covers the key findings from the publication and places the results into clinical context
Watch the video for key takeaways, and download the infographic for a quick reference
Access the LEAP-012 publication here: Kudo M, et al. Lancet. 2025;405:203-215
Clinical takeaways
- LEAP-012 further supports the approach of combining locoregional therapies with IO-based therapies
- Locoregional therapies combined with IO-based therapies should be more frequently considered in clinical practice, particularly for patients with intermediate HCC to downstage or downsize the disease
- Future directions include additional data on overall survival and quality of life, to further define the long-term benefits and clinical applicability









 Downloadable
Downloadable  20 MIN
20 MIN
 Feb 2026
Feb 2026 








